Background: Most patients (pts) with histone-lysine N-methyltransferase 2A (KMT2A)-rearranged (KMT2Ar) acute leukemia, relapse after conventional chemotherapy and hematopoietic stem cell transplant (HSCT). Remission rates after relapse (complete remission [CR], 5%) and median overall survival (2.4 mo) in ≥2nd salvage therapies in adults remain low (Blood Cancer J. 2021;11[9]:162). In KMT2Ar leukemia, interaction of menin with KMT2A fusion proteins is a key driver of leukemogenesis. However, no therapies targeting the menin-KMT2A interaction have been approved. Revumenib (SNDX-5613; rev), a small-molecule inhibitor of menin-KMT2A interactions, demonstrated preliminary efficacy and safety in a phase 1 study of R/R KMT2Ar and nucleophosmin 1-mutated (NPM1m) acute leukemias. We report topline efficacy and safety for pts with R/R KMT2Ar acute leukemia treated with rev in a pivotal phase 2 study (AUGMENT-101; NCT04065399).
Methods: Pts aged ≥30 days with R/R KMT2Ar acute leukemia were enrolled in cohort A (acute lymphoblastic leukemia [ALL]/mixed phenotype acute leukemia [MPAL]) and B (acute myeloid leukemia [AML]); cohort C continues to enroll pts with NPM1m and is not included in this analysis. Pts received rev (163 mg or 95 mg/m2 if body weight <40 kg) q12h with a strong cytochrome P450 3A4 inhibitor orally in 28-day cycles. Treatment continued until unacceptable toxicity or lack of at least morphological leukemia-free state (MLFS) after 4 cycles of treatment. Phase 2 primary objectives were safety and tolerability of rev and CR+CR with partial hematologic recovery (CRh) rate. Key secondary endpoints included composite CR rate (CRc, CR+CRh+CR with incomplete platelet recovery [CRp]+CR with incomplete count recovery [CRi]) and overall response rate (ORR, CRc+MLFS+partial remission). A planned interim analysis (IA) of pooled adult and pediatric pts with KMT2Ar acute leukemia was conducted.
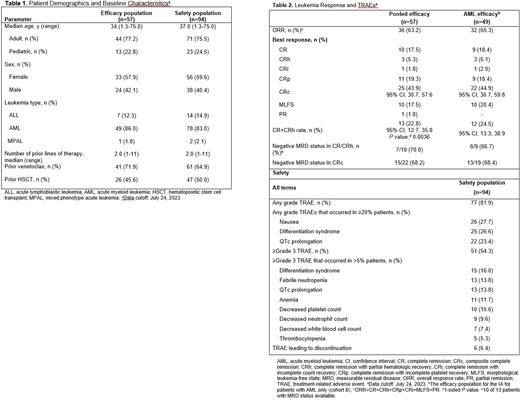
Results:As of July 24, 2023, 94 pts with R/R KMT2Ar acute leukemia received ≥1 dose of study drug and were included in the safety analysis (Table 1). Median age was 37.0 y (range, 1.3-75.0). Of the 94 pts, 23 (24.5%) were aged <18 y and 13 (13.8%) were aged ≥65 y. Seventy-eight (83%) had AML and 16 (17.0%) had ALL or MPAL. Over half of pts were female; 17.5% of pts were non-White. Pts were heavily pretreated (median 2 [range, 1-11] prior lines of therapy), with 41 pts (43.6%) having received ≥3 prior lines. Fifty-four pts (57.4%) had refractory relapse disease (unresponsive to most recent salvage treatment), and 47 pts (50%) had prior HSCT. Treatment-related adverse events (TRAEs) were reported in 81.9% of the safety population. Most common TRAEs (≥20%) were nausea (27.7%), differentiation syndrome (26.6%), and QTc prolongation (23.4%). Grade ≥3 TRAEs were observed in 51 pts (54.3%), the most common being differentiation syndrome (16.0%), febrile neutropenia (13.8%), and QTc prolongation (13.8%) (Table 2). Overall, 6.4% of pts discontinued therapy due to TRAEs; none discontinued rev due to differentiation syndrome or QTc prolongation.
The pooled efficacy population for the IA (n=57) included all phase 2 pts with centrally confirmed KMT2Ar and ≥5% blasts in bone marrow at baseline who had received ≥1 dose of study drug and started treatment on or before the 38th adult AML efficacy evaluable patient. The IA was specified to occur when the 57 patients have had the opportunity to be followed for 6 months. After median follow-up of 6.1 mo, 13 pts (22.8% [95% confidence interval (CI), 12.7-35.8]) achieved CR+CRh, surpassing the predefined IA efficacy boundary for the pooled KMT2Ar population; CR+CRh rate was similar in adult and pediatric pts. Median duration of CR+CRh was 6.4 mo (95% CI, 3.4-not reached). CRc was 43.9% (95% CI, 30.7-57.6); ORR was 63.2% (95% CI, 49.3-75.6). Most pts with a CR or CRh response, and for whom measurable residual disease (MRD) status was reported, achieved MRD negativity (7/10, 70.0%); most pts with CRc and MRD reported also achieved MRD negativity (15/22, 68.2%). Fourteen of 36 responders (38.9%) proceeded to HSCT, with half resuming rev post HSCT.
Conclusions: Rev demonstrated clinically meaningful results in a heavily pretreated KMT2Ar population, including high ORR and rates of MRD negativity and subsequent HSCT. At IA, this pivotal study met its primary endpoint and the KMT2Ar cohorts were stopped early for efficacy.
Disclosures
Aldoss:Takeda: Consultancy; Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Jazz: Consultancy; Sobi: Consultancy; KiTE: Consultancy. Issa:Merck: Research Funding; Celgene: Research Funding; Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; NuProbe: Consultancy; Syndax: Research Funding. Thirman:Syndax: Research Funding; Nurix: Research Funding; AbbVie: Research Funding; Merck: Research Funding; AbbVie: Honoraria. DiPersio:Bioline: Consultancy; Rivervest: Consultancy; Macrogenics: Research Funding; Vertex: Consultancy; Magenta: Current holder of stock options in a privately-held company, Other: Ownership Investment, Patents & Royalties; WUGEN: Current holder of stock options in a privately-held company, Other: Ownership Investment, Patents & Royalties, Research Funding. Blachly:Astellas: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Leukemia Diagnostic Device: Patents & Royalties: Being prosecuted; Epigenetic classification of leukemia: Patents & Royalties: PCT conversion filed. Mannis:Abbvie: Consultancy; Agios: Consultancy; Macrogenics: Honoraria; Astellas: Consultancy; BMS/Celgene: Consultancy; Genentech: Consultancy; Stemline: Consultancy. Perl:Daiichi-Sankyo: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Bayer: Research Funding; FujiFilm: Research Funding; Syndax: Research Funding; Forma: Consultancy; Foghorn: Consultancy; Beat AML: Other: Participation on a Data Safety Monitoring Board or Advisory Board; BerGen Bio: Honoraria; Genentech: Honoraria; Immunogen: Honoraria; BMS: Honoraria; Aptose: Honoraria; Rigel: Honoraria; Actinium: Honoraria. Dickens:Syndax: Research Funding; Amgen: Honoraria; American Academy of Pediatrics: Membership on an entity’s Board of Directors or advisory committees; American Society of Pediatric Hematology Oncology: Membership on an entity’s Board of Directors or advisory committees; Tempus Inc.: Honoraria; Iowa Cancer Consortium: Membership on an entity’s Board of Directors or advisory committees. McMahon:Syros Pharmaceuticals: Research Funding; Kura Oncology: Membership on an entity’s Board of Directors or advisory committees; Arcellx: Membership on an entity’s Board of Directors or advisory committees; Syndax Pharmaceuticals: Research Funding. Traer:Rigel: Membership on an entity’s Board of Directors or advisory committees; Servier: Membership on an entity’s Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity’s Board of Directors or advisory committees; Prelude Therapeutics: Research Funding; Schrodinger: Research Funding; Incyte: Research Funding; Astra-Zeneca: Research Funding. Zwaan:Kura Oncology: Consultancy; BMS: Consultancy; Novartis: Consultancy; Gilead: Consultancy; Incyte: Consultancy; Novartis: Membership on an entity’s Board of Directors or advisory committees; Sanofi: Membership on an entity’s Board of Directors or advisory committees; Pfizer: Research Funding; Abbvie: Research Funding; Takeda: Research Funding; Jazz: Research Funding. Grove:Abbvie: Consultancy; Astellas: Consultancy, Honoraria; Otsuka Australia: Consultancy. Stone:Abbvie: Consultancy. Shami:Chimerix: Research Funding; Amgen: Research Funding; Abcuro: Research Funding; Ono: Research Funding; BMS: Membership on an entity’s Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity’s Board of Directors or advisory committees; Gilead: Membership on an entity’s Board of Directors or advisory committees; Takeda: Membership on an entity’s Board of Directors or advisory committees; RJH Biosciences: Membership on an entity’s Board of Directors or advisory committees; JSK Therapeutics: Current holder of stock options in a privately-held company. Mantzaris:Kite, a Gilead company: Honoraria. Greenwood:Amgen: Honoraria, Other: Drug supply for a trial; Servier: Honoraria, Other: Assay costs, shipping costs - trial related; Jazz: Honoraria. Shukla:Syndax: Membership on an entity’s Board of Directors or advisory committees. Gu:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Bagley:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Madigan:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Nguyen:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. McNeer:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company; Astrazeneca: Research Funding. Stein:Eisai: Research Funding; Bristol Myers Squib: Consultancy, Research Funding; Novartis: Consultancy; PinotBio: Consultancy; Janssen: Consultancy; Agios: Consultancy; Jazz: Consultancy; Menarini: Consultancy; Genentech: Consultancy; Genesis: Consultancy; Abbvie: Consultancy; Neoleukin: Consultancy; Gilead: Consultancy; Syndax: Consultancy; OnCusp: Consultancy; CTI Biopharma: Consultancy; Foghorn: Consultancy; Servier: Consultancy; Calithera: Consultancy; Daiichi: Consultancy; Aptose: Consultancy; Syros: Consultancy; Astellas: Consultancy; Ono Pharma: Consultancy; Blueprint: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal